Table of Contents
How does an Aluminum air battery work?
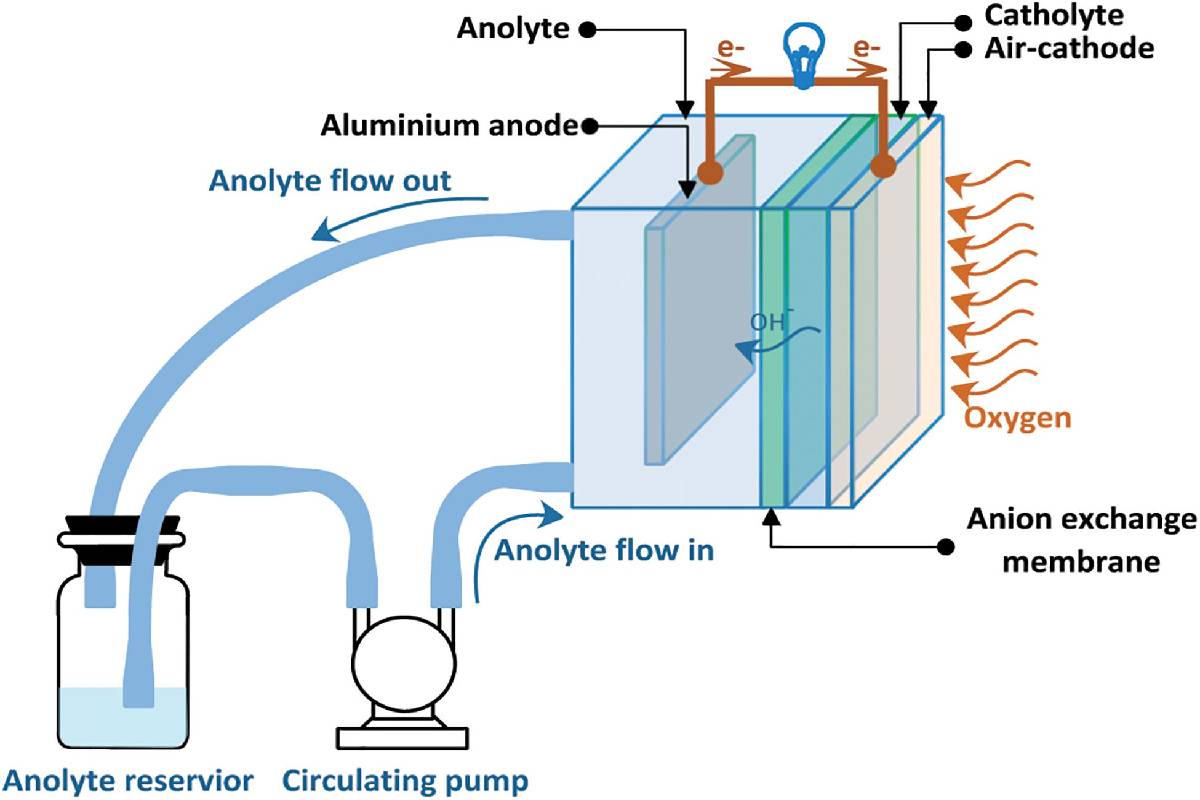
An Aluminum-air battery or Al-air battery produces electricity from the reaction of oxygen in the air with Aluminum.
It has one of the highest energy densities of all cells, but it is not yet widely used.
And also, due to problems with the high cost of the anode and the by-product removal.
When using traditional electrolytes, which has restricted its use to applications, mainly military.
However, an electric vehicle with aluminum batteries has the potential for up to eight times the lithium-ion battery range, with a significantly lower total weight.
https://www.dyifo.com/
Are Aluminum-air batteries rechargeable?
- Aluminum-air batteries are primary cells, that is, not rechargeable.
- Once the aluminum anode is consumed, by reaction with atmospheric oxygen at a cathode immersed in a water-based electrolyte to form hydrated aluminum oxide, the battery will stop producing electricity.
- However, it is possible to recharge the battery mechanically with new aluminum anodes made from recycling hydrated aluminum oxide.
- This recycling would be essential if aluminum-air batteries were to become widely adopted.
Aluminum-air battery powered vehicles
- Aluminum-powered vehicles have been the subject of debate for a few decades.
- Hybridization reduces costs, and road tests of an aluminum-air / lead-acid hybrid battery in an electric vehicle recorded in 1989.
- A plug-in hybrid minivan, powered by one of these batteries, was demonstrated in Ontario in 1990.
In March 2013, Phinergy released a demonstration video of an electric car using aluminum-air cells that moved for 330 kilometres using a separate cathode and potassium hydroxide. - On May 27, 2013, Israel’s Channel 10, on its evening news broadcast, showed a driving car with Phinergy batteries on its back, being “fed” with “pure” drinking water.
- Claiming that it had 2,000 kilometres of operation for a battery before replacing the aluminum anodes was necessary.
Are Aluminum-Air batteries the future?
- Smartphones, tablets, laptops, cameras, music players, household appliances, and a myriad of more or less common devices in our lives need a battery to work.
- That is why this field is advancing at great speed, and there are many investigations to make batteries smaller and more durable.
- We want to give you our vision of a type of battery that seems to be the most immediate future and represents a revolution in Aluminum-Air batteries.
- Currently, three companies are researching this technology with great expectations.
- In this type of battery, Aluminum’s anode, the cathode replace by air, while the electrolyte becomes simply an aqueous solution.
- In reality, this type of technology uses for military purposes, then; Why do we say they could be the batteries of the future? Let’s see its advantages compared to current Lithium batteries.
Advantages of Aluminum-Air batteries
- Thanks to their very high energy density, they have eight times more capacity than current batteries
- They have the same weight and volume as Li-ion
- Aluminum is an abundant metal and cheaper than Lithium
- Its price would be three times lower
- They are safer since they do not have a risk of explosion
- Their autonomy is one of the leading candidates to incorporate into electric cars and photovoltaic energy storage in self-sufficient homes.
Although for this it would be necessary to improve some of its weaknesses. - They are not all advantages for this emerging technology.
Disadvantages of Aluminum-Air batteries
- Its useful life is not known
- They are difficult to recharge. The aluminum anode rusts and is unusable, making it necessary to replace the metal
- As you can see, replacing the anode with a new one is an excellent point against these batteries.
- However, manufacturers developing batteries for which only ionized water would have to recharge.
- Another solution could a removable battery replaced by a new one, leaving the exhausted one at the service station.